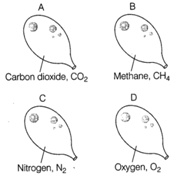
Answer:
Balloon A Molecular mass \[C{{O}_{2}}=12+16\times 2\] \[=12+32=44\,\text{u}\] Balloon B Molecular mass \[C{{H}_{4}}=12+1\times 4\] \[=12+4=28\,\text{u}\] Balloon C Molecular mass \[{{N}_{2}}=14\times 2=28\,\text{u}\] Balloon D Molecular mass \[{{O}_{2}}=16\times 2=32\,\text{u}\,\] Methane is the least dense of all as it has the lowest relative molecular mass. So, balloon B would go down most quickly. Remember lighter gas diffuses faster as compared to a heavier gas.
You need to login to perform this action.
You will be redirected in
3 sec
