Answer:
(a) Atomic number =12
Mass number = 26
Electronic configuration = 2, 8, 2
Atomic structure of X
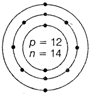 Nuclear composition
Number of protons =12
Number of neutrons =26-12=14
Valency = 2
Because it can donate 2 electrons easily to complete its
octet and become stable.
(b) Valency of the element 'Y 'would be 1, i.e., it can gain
1 electron to become stable. When it combines with the element 'X' of valency
2, the compound formed will be
Nuclear composition
Number of protons =12
Number of neutrons =26-12=14
Valency = 2
Because it can donate 2 electrons easily to complete its
octet and become stable.
(b) Valency of the element 'Y 'would be 1, i.e., it can gain
1 electron to become stable. When it combines with the element 'X' of valency
2, the compound formed will be ![]() .
.
![]() Formula of the compound would be
Formula of the compound would be ![]()
You need to login to perform this action.
You will be redirected in
3 sec
