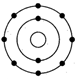
 (i) What is wrong with this structure of atom?
(ii) Draw a correct representation of this atom.
(iii) Write the chemical formula of the chloride of this element.
(i) What is wrong with this structure of atom?
(ii) Draw a correct representation of this atom.
(iii) Write the chemical formula of the chloride of this element.
Answer:
(i) Na has three shells K, L and M but here only 2 shell
are given. Further L shell cannot have more than 8 electrons but here 9
electrons are given.
(ii) The correct structure is
 (iii) As Na has 1 valence electrons, thus it has a valency
of +1 and chlorine has a valency of-1
Hence, the formula of its chloride is AC1.
(a) Nucleus
(b) Oxygen and calcium
(c)
(iii) As Na has 1 valence electrons, thus it has a valency
of +1 and chlorine has a valency of-1
Hence, the formula of its chloride is AC1.
(a) Nucleus
(b) Oxygen and calcium
(c) ![]()

| Element | Atomic number | Proton | Electron | Neutron | Mass number |
| A B C | 17 14 9 | 17 14 9 | 17 14 9 | 18 14 19-9=10 | 17+18=35 14+14=28 19 |
| Particles | Atomic number | Mass number |
| A B C D | 3 9 8 9 | 3 = 4 =7 9 + 8 = 17 8 + 8 = 16 8 + 10 = 18 |
You need to login to perform this action.
You will be redirected in
3 sec
