A) If both assertion and reason are true and the reason is the correct explanation of the assertion.
B) If both assertion and reason are true but reason is not the correct explanation of the assertion.
C) If assertion is true but reason is false.
D) If the assertion and reason both are false.
E) If assertion is false but reason is true.
Correct Answer: B
Solution :
As we know, in thermodynamic processes work done = Area covered by P-V diagram with volume axis. Hence, according to following graph. (Area)1 < (Area)2 Þ Wadi < Wiso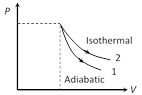 Also in isothermal changes temperature remains same but in adiabatic changes temperature also changes.
Also in isothermal changes temperature remains same but in adiabatic changes temperature also changes.
You need to login to perform this action.
You will be redirected in
3 sec
