A) \[n=2\text{ to} n=1\]
B) \[n=1\text{ to }n=2\]
C) \[n=2\text{ }\,\text{to}\,\,n=5\]
D) \[n=5\text{ to }n=2\]
Correct Answer: D
Solution :
\[\because \ {{E}_{2}}<{{E}_{1}}\]Þ \[{{\lambda }_{2}}>{{\lambda }_{1}}\]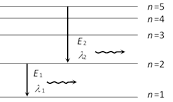
You need to login to perform this action.
You will be redirected in
3 sec
