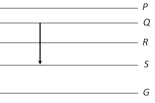
A) P to Q
B) Q to R
C) R to S
D) R to G
Correct Answer: D
Solution :
If E is the energy radiated in transition then \[{{E}_{R\to G}}>{{E}_{Q\to S}}>{{E}_{R\to S}}>{{E}_{Q\to R}}>{{E}_{P\to Q}}\] For getting blue line energy radiated should be maximum \[\left( E\propto \frac{1}{\lambda } \right)\]. Hence (d) is the correct option.You need to login to perform this action.
You will be redirected in
3 sec
