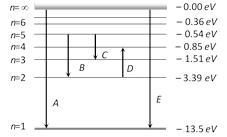
A) First member of Lyman series, third spectral line of Balmer series and the second spectral line of Paschen series
B) Ionization potential of hydrogen, second spectral line of Balmer series and third spectral line of Paschen series
C) Series limit of Lyman series, third spectral line of Balmer series and second spectral line of Paschen series
D) Series limit of Lyman series, second spectral line of Balmer series and third spectral line of Paschen series
Correct Answer: C
Solution :
Transition A (n = ¥ to 1) : Series limit of Lyman series Transition B (n = 5 to n = 2) : Third spectral line of Balmer series Transition C (n = 5 to n = 3) : Second spectral line of Paschen seriesYou need to login to perform this action.
You will be redirected in
3 sec
