A) \[1.602\times {{10}^{-14}}\,J\]
B) \[1.619\times {{10}^{-16}}\,J\]
C) \[1.632\times {{10}^{-18}}J\]
D) \[1.656\times {{10}^{-20}}J\]
Correct Answer: C
Solution :
Energy to excite the \[{{e}^{-}}\] from \[n=1\] to \[n=2\]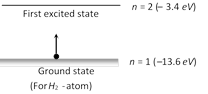 \[E=-3.4-(-13.6)=10.2\,eV=10.2\times 1.6\times {{10}^{-19}}\] \[=1.632\times {{10}^{-18}}\,J\]
\[E=-3.4-(-13.6)=10.2\,eV=10.2\times 1.6\times {{10}^{-19}}\] \[=1.632\times {{10}^{-18}}\,J\]
You need to login to perform this action.
You will be redirected in
3 sec
