A) 3100
B) 3200
C) 3600
D) 4200
Correct Answer: C
Solution :
Ice (0°C) converts into steam (100°C) in following three steps.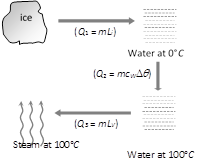 Total heat required \[Q={{Q}_{1}}+{{Q}_{2}}+{{Q}_{3}}\] \[=5\times 80+5\times 1\times (100-0)+5\times 540=3600\,cal\]
Total heat required \[Q={{Q}_{1}}+{{Q}_{2}}+{{Q}_{3}}\] \[=5\times 80+5\times 1\times (100-0)+5\times 540=3600\,cal\]
You need to login to perform this action.
You will be redirected in
3 sec
