A) \[2.0\times {{10}^{10}}\]N
B) \[2.0\times {{10}^{4}}\]N
C) \[2.0\times {{10}^{8}}\]N
D) \[2.0\times {{10}^{6}}\]N
Correct Answer: C
Solution :
Number of atoms in given mass \[=\frac{10}{63.5}\times 6.02\times {{10}^{23}}\] = 9.48 ´ 1022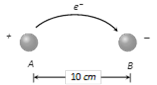 Transfer of electron between balls \[=\frac{9.48\times {{10}^{22}}}{{{10}^{6}}}\] = 9.48 ´ 1016 Hence magnitude of charge gained by each ball. Q = 9.48 ´ 1016 ´ 1.6 ´ 10?19 = 0.015 C Force of attraction between the balls \[F=9\times {{10}^{9}}\times \frac{{{(0.015)}^{2}}}{{{(0.1)}^{2}}}=2\times {{10}^{8}}N.\]
Transfer of electron between balls \[=\frac{9.48\times {{10}^{22}}}{{{10}^{6}}}\] = 9.48 ´ 1016 Hence magnitude of charge gained by each ball. Q = 9.48 ´ 1016 ´ 1.6 ´ 10?19 = 0.015 C Force of attraction between the balls \[F=9\times {{10}^{9}}\times \frac{{{(0.015)}^{2}}}{{{(0.1)}^{2}}}=2\times {{10}^{8}}N.\]
You need to login to perform this action.
You will be redirected in
3 sec
