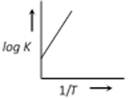
A)
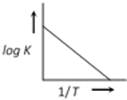
B)
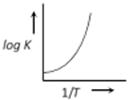
C)
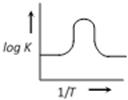
D)
Correct Answer: B
Solution :
A graph plotted between \[\log k\,\,\text{Vs}\,\frac{1}{T}\] for calculating activation energy is shown as from Arrhenius equation \[\log \,\,k=\log \,\,A-\frac{{{E}_{A}}}{2.303\,\,RT}\]
\[\log \,\,k=\log \,\,A-\frac{{{E}_{A}}}{2.303\,\,RT}\]
You need to login to perform this action.
You will be redirected in
3 sec
