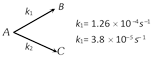 The percentage distribution of B and C are [Kerala PMT 2004]
The percentage distribution of B and C are [Kerala PMT 2004]
A) 75% B and 25% C
B) 80% B and 20% C
C) 60% B and 40% C
D) 90% B and 10% C
E) 76.83% B and 23.17% C
Correct Answer: E
Solution :
% distribution of \[B=\frac{{{K}_{1}}}{{{K}_{1}}+{{K}_{2}}}\times 100\] \[=\frac{1.26\times {{10}^{-4}}}{1.26\times {{10}^{-4}}+3.8\times {{10}^{-4}}}\times 100\] \[B%=76.83%\] \[%\text{Distribution of }C=\frac{{{K}_{2}}}{{{K}_{1}}+{{K}_{2}}}\times 100\] \[=\frac{3.8\times {{10}^{-4}}}{1.26\times {{10}^{-4}}+3.8\times {{10}^{-4}}}\times 100\] \[C%=23.17%\]You need to login to perform this action.
You will be redirected in
3 sec
