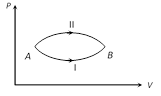
A) \[\Delta {{U}_{\text{II}}}>\Delta {{U}_{\text{I}}}\]
B) \[\Delta {{U}_{\text{II}}}<\Delta {{U}_{\text{I}}}\]
C) \[\Delta {{U}_{\text{I}}}=\Delta {{U}_{\text{II}}}\]
D) Relation between \[\Delta {{U}_{\text{I}}}\] and \[\Delta {{U}_{\text{II}}}\] can not be determined
Correct Answer: C
Solution :
As internal energy is a point function therefore change in internal energy does not depends upon the path followed i.e. \[\Delta {{U}_{\text{I}}}=\Delta {{U}_{\text{II}}}\]You need to login to perform this action.
You will be redirected in
3 sec
