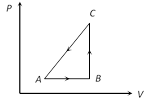
A) 34 J
B) 70 J
C) 84 J
D) 134 J
Correct Answer: D
Solution :
Heat given DQ \[=20\,cal=20\times 4.2\,=84\,J\]. Work done DW = ? 50 J [As process is anticlockwise] By first law of thermodynamics Þ \[\Delta U=\Delta Q-\Delta W=84-(-\,50)\,=134J\]You need to login to perform this action.
You will be redirected in
3 sec
