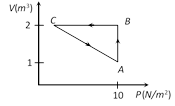
A) ? 5 J
B) ? 10 J
C) ? 15 J
D) ? 20 J
Correct Answer: A
Solution :
For cyclic process. Total work done \[={{W}_{AB}}+{{W}_{BC}}+{{W}_{CA}}\] DWAB = PDV = 10(2 ? 1) = 10J and DWBC =0 (as V = constant) From FLOT, DQ = DU + DW DU = 0 (Process ABCA is cyclic) Þ DQ = DWAB + DWBC + DWCA Þ 5 = 10 + 0 + DWCA Þ DWCA = ? 5 JYou need to login to perform this action.
You will be redirected in
3 sec
