A) \[0,\,R{{T}_{2}}\ln \left( \frac{{{V}_{1}}}{{{V}_{2}}} \right)\,,\,R\,({{T}_{1}}-{{T}_{2}})\]
B) \[R({{T}_{1}}-{{T}_{2}}),\,0,\,R{{T}_{1}}\ln \frac{{{V}_{1}}}{{{V}_{2}}}\]
C) \[0,\,R{{T}_{2}}\ln \left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\,,\,R\,({{T}_{1}}-{{T}_{2}})\]
D) \[0,\,R{{T}_{2}}\ln \left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\,,\,R\,({{T}_{2}}-{{T}_{1}})\]
Correct Answer: C
Solution :
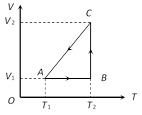
Process AB is isochoric, \ \[{{W}_{AB}}=P\,\Delta V=0\] Process BC is isothermal \ \[{{W}_{BC}}=R{{T}_{2}}.\ln \left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\] Process CA is isobaric \ \[{{W}_{CA}}=-\,P\Delta V\]\[=-\,R\Delta T\]\[=-\,R({{T}_{1}}-{{T}_{2}})\]\[=R({{T}_{2}}-{{T}_{1}})\] (Negative sign is taken because of compression)You need to login to perform this action.
You will be redirected in
3 sec
