A) \[1/3\]
B) \[2/3\]
C) \[1/2\]
D) \[1/4\]
Correct Answer: A
Solution :
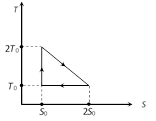
\[{{Q}_{1}}={{T}_{0}}{{S}_{0}}+\frac{1}{2}{{T}_{0}}{{S}_{0}}=\frac{3}{2}{{T}_{0}}{{S}_{0}}\] \[{{Q}_{2}}={{T}_{0}}{{S}_{0}}\]and \[{{Q}_{3}}=0\]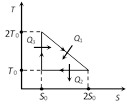 \[\eta =\frac{W}{{{Q}_{1}}}=\frac{{{Q}_{1}}-{{Q}_{2}}}{{{Q}_{1}}}\] \[=1-\frac{{{Q}_{2}}}{{{Q}_{1}}}=1-\frac{2}{3}=\frac{1}{3}\]
\[\eta =\frac{W}{{{Q}_{1}}}=\frac{{{Q}_{1}}-{{Q}_{2}}}{{{Q}_{1}}}\] \[=1-\frac{{{Q}_{2}}}{{{Q}_{1}}}=1-\frac{2}{3}=\frac{1}{3}\]
You need to login to perform this action.
You will be redirected in
3 sec
