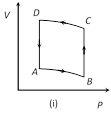

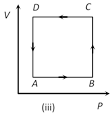
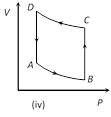
A) Positive in all cases (i) to (iv)
B) Positive in cases (i), (ii) and (iii) but zero in (iv) case
C) Negative in cases (i), (ii) and (iii) but zero in (iv) case
D) Zero in all four cases
Correct Answer: D
Solution :
In all given cases, process is cyclic and in cyclic process DU = 0.You need to login to perform this action.
You will be redirected in
3 sec
