A) \[\pi \times {{10}^{3}}J\]
B) \[\frac{\pi }{2}J\]
C) \[4\pi \times {{10}^{2}}J\]
D) \[\pi \,J\]
Correct Answer: B
Solution :
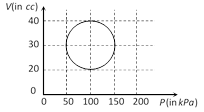
In cyclic process DQ = Work done = Area inside the closed curve. Treat the circle as an ellipse of area \[=\frac{\pi }{4}({{P}_{2}}-{{P}_{1}})\,({{V}_{2}}-{{V}_{1}})\] Þ \[\Delta Q=\frac{\pi }{4}\{(150-50)\times {{10}^{3}}\}=\frac{\pi }{2}J\]You need to login to perform this action.
You will be redirected in
3 sec
