A) \[7.5\times {{10}^{5}}\ joule\]
B) \[7.5\times {{10}^{5}}\ erg\]
C) \[12\times {{10}^{5}}\ joule\]
D) \[6\times {{10}^{5}}\ joule\]
Correct Answer: C
Solution :
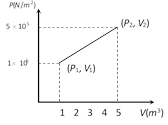
Work done = Area of PV graph (here trapezium) \[=\frac{1}{2}(1\times {{10}^{5}}+5\times {{10}^{5}})\times (5-1)=12\times {{10}^{5}}J\]You need to login to perform this action.
You will be redirected in
3 sec
