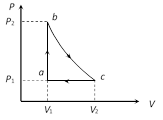
A) 4200 J
B) 5000 J
C) 9000 J
D) 9800 J
Correct Answer: D
Solution :
For path ab : \[{{(\Delta U)}_{ab}}=7000\ J\] By using \[\Delta U=\mu {{C}_{V}}\Delta T\] \[7000=\mu \times \frac{5}{2}R\times 700\Rightarrow \mu =0.48\] For path ca : \[{{(\Delta Q)}_{ca}}={{(\Delta U)}_{ca}}+{{(\Delta W)}_{ca}}\] ?.(i) \[\because \]\[{{(\Delta U)}_{ab}}+{{(\Delta U)}_{bc}}+{{(\Delta U)}_{ca}}=0\] \[\therefore \]\[7000+0+{{(\Delta U)}_{ca}}=0\Rightarrow {{(\Delta U)}_{ca}}=-7000\ J\] ?.(ii) Also \[{{(\Delta W)}_{ca}}={{P}_{1}}({{V}_{1}}-{{V}_{2}})=\mu R({{T}_{1}}-{{T}_{2}})\] \[=0.48\times 8.31\times (300-1000)=-2792.16\ J\] ?.(iii) on solving equations (i), (ii) and (iii) \[{{(\Delta Q)}_{ca}}=-7000-2792.16=-9792.16\ J\]\[=-9800\ J\]You need to login to perform this action.
You will be redirected in
3 sec
