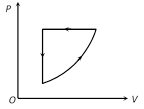
A) \[\Delta {{E}_{\operatorname{int}}}=0,\,Q<O\]
B) \[\Delta {{E}_{\operatorname{int}}}=0,\,Q>0\]
C) \[\Delta \,{{E}_{\operatorname{int}}}>0,\,Q<0\]
D) \[\Delta \,{{E}_{\operatorname{int}}}<0,\,Q>\,0\]
Correct Answer: A
Solution :
\[\Delta {{E}_{\text{int}}}=0\], for a complete cycle and for given cycle work done is negative, so from first law of thermodynamics Q will be negative i.e. \[Q<0\].You need to login to perform this action.
You will be redirected in
3 sec
