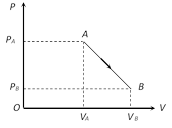
A) \[({{P}_{A}}-{{P}_{B}})({{V}_{B}}-{{V}_{A}})\]
B) \[\frac{1}{2}({{P}_{B}}-{{P}_{A}})({{V}_{B}}+{{V}_{A}})\]
C) \[\frac{1}{2}({{P}_{B}}-{{P}_{A}})({{V}_{B}}-{{V}_{A}})\]
D) \[\frac{1}{2}({{P}_{B}}+{{P}_{A}})({{V}_{B}}-{{V}_{A}})\]
Correct Answer: D
Solution :
W = Area bonded by the indicator diagram with V-axis) \[=\frac{1}{2}({{P}_{A}}+{{P}_{B}})\,({{V}_{B}}-{{V}_{A}})\]You need to login to perform this action.
You will be redirected in
3 sec
