A) 20 kJ
B) 30 kJ
C) 40 kJ
D) 60 kJ
Correct Answer: C
Solution :
Processes A to B and C to D are parts of straight line graphs of the form y = mx Also \[P=\frac{\mu R}{V}T\] (m = 6) Þ P µ T. So volume remains constant for the graphs AB and CD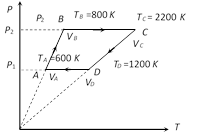 So no work is done during processes for A to B and C to D i.e., WAB = WCD = 0 and WBC = P2(VC ? VB) = mR (TC ? TB) = 6R (2200 ? 800) = 6R ´ 1400 J Also WDA = P1 (VA ? VD) = mR(TA ? TB) = 6R (600 ? 1200)= ? 6R ´ 600 J Hence work done in complete cycle W = WAB + WBC + WCD + WDA = 0 + 6R ´ 1400 + 0 ? 6R ´ 600 = 6R ´ 900 = 6 ´ 8.3 ´ 800 » 40 kJ
So no work is done during processes for A to B and C to D i.e., WAB = WCD = 0 and WBC = P2(VC ? VB) = mR (TC ? TB) = 6R (2200 ? 800) = 6R ´ 1400 J Also WDA = P1 (VA ? VD) = mR(TA ? TB) = 6R (600 ? 1200)= ? 6R ´ 600 J Hence work done in complete cycle W = WAB + WBC + WCD + WDA = 0 + 6R ´ 1400 + 0 ? 6R ´ 600 = 6R ´ 900 = 6 ´ 8.3 ´ 800 » 40 kJ
You need to login to perform this action.
You will be redirected in
3 sec
