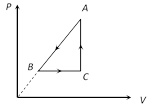
A) \[\Delta {{Q}_{A\to B}}\]= negative
B) \[\Delta {{U}_{B\to C}}\]= positive
C) \[\Delta {{W}_{CAB}}\]= negative
D) All of these
Correct Answer: D
Solution :
During process A to B, pressure and volume both are decreasing. Therefore, temperature and hence, internal energy of the gas will decrease (T µ PV) or \[\Delta {{U}_{A\to B}}=\]negative. Further \[\Delta {{W}_{A\to B}}\]is also negative as the volume of the gas is decreasing. Thus \[\Delta {{Q}_{A\to B}}\] is negative. In process B to C pressure of the gas is constant while volume is increasing. Hence temperature should increase or \[\Delta {{U}_{B\to C}}\]= positive. During C to A volume is constant while pressure is increasing. Therefore, temperature and hence, internal energy of the gas should increase or \[\Delta {{U}_{C\to A}}\]= positive. During process CAB volume of the gas is decreasing. Hence, work done by the gas is negative.You need to login to perform this action.
You will be redirected in
3 sec
