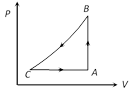
A) 1590 J
B) 1620 J
C) 1540 J
D) 1570 J
Correct Answer: A
Solution :
\[\Delta {{W}_{AB}}=0\] as V = constant \ \[\Delta {{Q}_{AB}}=\Delta {{U}_{AB}}=50J\] (Given) \[{{U}_{A}}=1500J\] \ \[{{U}_{B}}=(1500+50)J=1550J\] \[\Delta {{W}_{BC}}=-\Delta {{U}_{BC}}=-40J\] (Given) \ \[\Delta {{U}_{BC}}=40J\]\ \[{{U}_{C}}=(1550+40)J=1590J\]You need to login to perform this action.
You will be redirected in
3 sec
