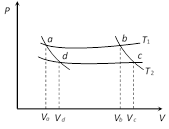
A) \[\frac{{{V}_{b}}}{{{V}_{c}}}\]
B) \[\frac{{{V}_{c}}}{{{V}_{b}}}\]
C) \[\frac{{{V}_{d}}}{{{V}_{a}}}\]
D) \[{{V}_{b}}{{V}_{c}}\]
Correct Answer: A
Solution :
For adiabatic process \[{{T}_{1}}V_{b}^{\gamma -1}\]= Constant For bc curve \[{{T}_{1}}V_{b}^{\gamma -1}={{T}_{2}}V_{c}^{\gamma -1}\] or \[\frac{{{T}_{2}}}{{{T}_{1}}}={{\left( \frac{{{V}_{b}}}{{{V}_{c}}} \right)}^{\gamma -1}}\] ?..(i) For ad curve \[{{T}_{1}}V_{a}^{\gamma -1}={{T}_{2}}V_{d}^{\gamma -1}\] or \[\frac{{{T}_{2}}}{{{T}_{1}}}={{\left( \frac{{{V}_{a}}}{{{V}_{d}}} \right)}^{\gamma -1}}\] ?..(ii) From equation (i) and (ii) \[\frac{{{V}_{b}}}{{{V}_{c}}}=\frac{{{V}_{a}}}{{{V}_{d}}}\]You need to login to perform this action.
You will be redirected in
3 sec
