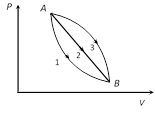
A) \[{{Q}_{1}}<{{Q}_{2}}<{{Q}_{3}}\]
B) \[{{Q}_{1}}<{{Q}_{2}}={{Q}_{3}}\]
C) \[{{Q}_{1}}={{Q}_{2}}>{{Q}_{3}}\]
D) \[{{Q}_{1}}>{{Q}_{2}}>{{Q}_{3}}\]
Correct Answer: A
Solution :
Initial and final states are same in all the process. Hence D U = 0; in each case. By FLOT; DQ = DW = Area enclosed by curve with volume axis. \[\because \] (Area)1 < (Area)2 < (Area)1 Þ Q1 < Q2 < Q3 .You need to login to perform this action.
You will be redirected in
3 sec
