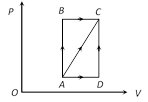
A) 560 J
B) 800 J
C) 600 J
D) 640 J
Correct Answer: A
Solution :
By adjoining graph \[{{W}_{AB}}=0\] and \[{{W}_{BC}}=8\times {{10}^{4}}[5-2]\times {{10}^{-3}}=240\,J\] \ \[{{W}_{AC}}={{W}_{AB}}+{{W}_{BC}}=0+240=240\,J\] Now, \[\Delta {{Q}_{AC}}=\Delta {{Q}_{AB}}+\Delta {{Q}_{BC}}=600+200=800\,J\] From FLOT \[\Delta {{Q}_{AC}}=\Delta {{U}_{AC}}+\Delta {{W}_{AC}}\] Þ \[800=\Delta {{U}_{AC}}+240\] Þ \[\Delta {{U}_{AC}}=560\,J.\]You need to login to perform this action.
You will be redirected in
3 sec
