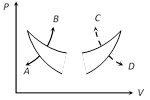
A) C and D respectively
B) D and C respectively
C) A and B respectively
D) B and A respectively
Correct Answer: C
Solution :
As we know that slope of isothermal and adiabatic curves are always negative and slope of adiabatic curve is always greater than that of isothermal curve Hence in the given graph curve A and B represents adiabatic and isothermal changes respectively.You need to login to perform this action.
You will be redirected in
3 sec
