A) \[{{H}_{2}}S<N{{H}_{3}}<Si{{H}_{4}}<B{{F}_{3}}\]
B) \[N{{H}_{3}}<{{H}_{2}}S<Si{{H}_{4}}<B{{F}_{3}}\]
C) \[{{H}_{2}}S<Si{{H}_{4}}<N{{H}_{3}}<B{{F}_{3}}\]
D) \[{{H}_{2}}S<N{{H}_{3}}<B{{F}_{3}}<Si{{H}_{4}}\]
Correct Answer: C
Solution :
The correct order of bond angle (Smallest first) is \[{{H}_{2}}S<N{{H}_{3}}<Si{{H}_{4}}<B{{F}_{3}}\] \[92.6{}^\circ <107{}^\circ <109{}^\circ 28'<120{}^\circ \]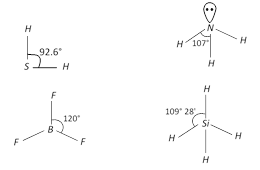
You need to login to perform this action.
You will be redirected in
3 sec
