A) \[s{{p}^{3}}\] hybridisation
B) \[s{{p}^{2}}\] hybridisation
C) \[sp\] hybridisation
D) \[{{d}^{2}}s{{p}^{3}}\] hybridization
Correct Answer: C
Solution :
\[{{C}_{\text{ground state}}}=2{{s}^{2}},2{{p}_{x}}^{1}{{p}_{y}}^{1}\]; \[{{C}_{\text{excited state }}}=2{{s}^{1}},2{{p}_{x}}^{1}{{p}_{y}}^{1}{{p}_{z}}^{1}\] \[{{O}_{\text{ground state }}}=2{{s}^{2}},2{{p}_{x}}^{2}{{p}_{y}}^{1}{{p}_{z}}^{1}\] In the formation of \[C{{O}_{2}}\] molecule, hybridization of orbitals of carbon occur only to a limited extent involving only one s and one p orbitals there is thus sp hybridisation of valence shell orbitals of the carbon atom resulting in the formation of two sp hybrid orbitals.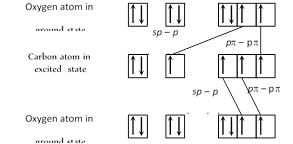
You need to login to perform this action.
You will be redirected in
3 sec
