A) Benzoicacid
B) Acetanilide
C) Aniline
D) Glycine
Correct Answer: D
Solution :
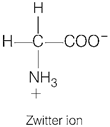
[d]| Ion containing positive as well as negative charge is called Zwitter ion. |
| Among the given options, only glycine \[({{H}_{2}}NC{{H}_{2}}COOH)\] is an amino acid which contains both acidic (acquiring negative charge) and basic group (acquiring positive charge). |
| Therefore, glycine can form a Zwitter ion. It is because glycine behave like salt rather than simple amines or carboxylic acids. In aqueous solution, the carboxyl group can lose a proton and amino group can accept a proton giving rise to a dipolar ion known as Zwitter ion. |
 |
You need to login to perform this action.
You will be redirected in
3 sec
