| The given graph shows the vapour pressure- temperature curves for some liquids. |
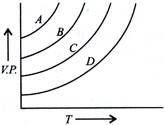 |
| Liquids A, B, C and D respectively are |
A) diethyl ether, acetone, ethyl alcohol, water
B) acetone, ethyl alcohol, diethyl ether, water
C) water, ethyl alcohol, acetone, diethyl ether
D) ethyl alcohol, acetone, diethyl ether, water.
Correct Answer: A
Solution :
The vapour pressure increases with increase in intermolecular forces. When the forces are weak, the liquid has high volatility and maximum vapour pressure. Diethyl ether has highest vapour pressure while water has lowest vapour pressure.You need to login to perform this action.
You will be redirected in
3 sec
