| In the given crystal structure what should be the cation X which replaces \[N{{a}^{+}}\]to create to cation vacancy? |
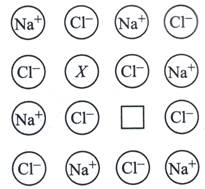 |
A) \[S{{r}^{2+}}\]
B) \[{{K}^{+}}\]
C) \[L{{i}^{+}}\]
D) \[B{{r}^{-}}\]
Correct Answer: A
Solution :
Each \[S{{r}^{2+}}\] in the NaCl crystal replaces two \[N{{a}^{+}}\] ions. It occupies the site of one ion and the other remains vacant creating a cation vacancy.You need to login to perform this action.
You will be redirected in
3 sec
