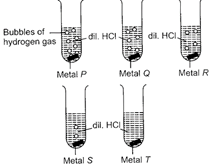
A)
P Q R S T Zn Al Mg Cu Fe
B)
P Q R S T Mg Al Zn Fe Cu
C)
P Q R S T Al Zn Mg Fe Cu
D)
P Q R S T Fe Zn Mg Al Cu
Correct Answer: B
Solution :
In reactivity series, the order of reactivity of given metals is as follows: Mg > Al > Zn > Fe > Cu Thus, rate of evolution of Hz gas in case of Mg is maximum followed by Al, Zn and then Fe. Cu does not react with dil. \[HCl,\] so no evolution of \[{{H}_{2}}\]gas is observed.You need to login to perform this action.
You will be redirected in
3 sec
