A) I, II
B) II, III
C) I, II, III
D) I, III
Correct Answer: C
Solution :
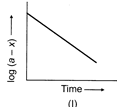
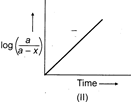
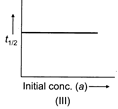
[c] All the three plots are correct. (I) \[\log (a-x)\], where \[(a-x)\] is remaining concentration, decreases with passage of time. (II) \[k=\frac{2.303}{t}{{t}_{{{\log }_{10}}}}\left( \frac{a}{a-x} \right)\] \[\log \left( \frac{a}{a-x} \right)=\frac{k}{2.303}\times t\] Plot of \[\log \left( \frac{a}{a-x} \right)\] against 't' gives straight line through origin. (III) \[{{t}_{1/2}}=\frac{0.693}{k};\]Half-life of first order reaction does not depend on initial concentration. Thus, graph of \[{{t}_{1/2}}\] against 'a' gives horizontal straight line.You need to login to perform this action.
You will be redirected in
3 sec
