A) 0.288
B) 0.577
C) 1.154
D) none of these
Correct Answer: C
Solution :
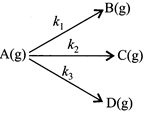
[c] Overall rate constant \[k={{k}_{1}}+{{k}_{2}}+{{k}_{3}}=6.93\times {{10}^{-3}}\] \[{{t}_{1/2}}=\frac{0.693}{6.93\times {{10}^{-3}}}=100\sec ;\] After half-life, \[{{P}_{B}}+{{P}_{C}}+{{P}_{D}}=4atm\] \[\frac{{{P}_{B}}}{{{P}_{B}}+{{P}_{C}}+{{P}_{D}}}=\frac{{{k}_{1}}}{{{k}_{1}}+{{k}_{2}}+{{k}_{3}}}=\frac{200}{693}\] \[{{P}_{B}}=4\times \frac{200}{639}=1.154atm\]You need to login to perform this action.
You will be redirected in
3 sec
