A) \[W=-2\,{{P}_{0}}{{V}_{0}}ln\,2\]
B) \[W=-2\,{{P}_{0}}{{V}_{0}}(1+ln\,2)\]
C) \[W=-\,{{P}_{0}}{{V}_{0}}(1+ln\,2)\]
D) \[W=-\,{{P}_{0}}{{V}_{0}}ln\,2\]
Correct Answer: A
Solution :
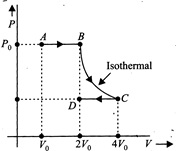
[a] At A and D the temperatures of the gas will be equal, so \[\Delta E=0\], \[{{W}_{AB}}=-{{P}_{0}}(2{{V}_{0}}-{{V}_{0}})=-{{P}_{0}}{{V}_{0}}\] \[{{W}_{BC}}=-{{P}_{0}}\times 2{{V}_{0}}\] ln \[\left( \frac{4{{V}_{0}}}{2{{V}_{0}}} \right)=-2{{P}_{0}}{{V}_{0}}\] ln 2 \[{{W}_{CD}}=\frac{{{P}_{0}}}{2}(4{{V}_{0}}-2{{V}_{0}})={{P}_{0}}{{V}_{0}}\] Now \[W={{W}_{AB}}+{{W}_{Bc}}+{{W}_{CD}}=-{{P}_{0}}{{V}_{0}}-{{P}_{0}}{{V}_{0}}\] ln \[2+{{P}_{0}}{{V}_{0}}\]\[=-2{{P}_{0}}{{V}_{0}}\] ln 2You need to login to perform this action.
You will be redirected in
3 sec
