A) tetrahedral and tetrahedral
B) square planar and square planar
C) tetrahedral and square planar
D) square planar and tetrahedral
Correct Answer: C
Solution :
[c] Un the given complex, \[NiC{{l}_{2}}{{\{P{{({{C}_{2}}{{H}_{5}})}_{2}}({{C}_{6}}{{H}_{5}})\}}_{2}}\] nickel is in +2 oxidation state and the ground state electronic configuration of \[N{{i}^{2+}}\]ions in free gaseous state is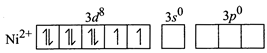 For the given four coordinated complex to be paramagnetic, it must possess unpaired electrons in the valence shell. To satisfy this condition, four lone pairs form the four ligands occupy the four \[s{{p}^{3}}\text{-}\]hybrid orbitals as:
For the given four coordinated complex to be paramagnetic, it must possess unpaired electrons in the valence shell. To satisfy this condition, four lone pairs form the four ligands occupy the four \[s{{p}^{3}}\text{-}\]hybrid orbitals as: 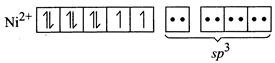 Therefore, geometry of paramagnetic complex must be tetrahedral. On the other-hand, for complex to be diamagnetic, there should not be any unpaired electrons in the valence shell. This condition can be fulfilled by pairing electrons of 3d-orbitals against Hund?s rule as
Therefore, geometry of paramagnetic complex must be tetrahedral. On the other-hand, for complex to be diamagnetic, there should not be any unpaired electrons in the valence shell. This condition can be fulfilled by pairing electrons of 3d-orbitals against Hund?s rule as 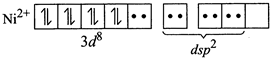 The above electronic arrangement gives \[ds{{p}^{2}}\]-hybridisation and therefore, square planar geometry to the complex.
The above electronic arrangement gives \[ds{{p}^{2}}\]-hybridisation and therefore, square planar geometry to the complex.
You need to login to perform this action.
You will be redirected in
3 sec
