A) \[{{[Sc{{({{H}_{2}}O)}_{6}}]}^{3+}}\] and \[{{[Ti{{({{H}_{2}}O)}_{6}}]}^{3+}}\] both are colourless.
B) \[[Co{{(N{{H}_{3}})}_{4}}B{{r}_{2}}]Cl\] shows ionization isomers and geometrical isomers.
C) \[[Pd{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{2}}]\] is square planar and shows geometrical as well as linkage isomers.
D) Both [b] and [c] are correct.
Correct Answer: D
Solution :
[d] (1)\[S{{c}^{3+}}-{{[Ar]}^{18}}3{{d}^{0}}4{{s}^{0}};\]there is no unpaired electron in d-orbitals so no d-d transition takes place and the complex is colourless. \[T{{i}^{3+}}-{{[Ar]}^{18}}3{{d}^{1}}4{{s}^{0}};\] it has one unpaired electron so d-d transition of electron form \[{{t}_{2g}}\] level to empty level takes place and thus the complex is colored. (2) \[[Co{{(N{{H}_{3}})}_{4}}B{{r}_{2}}]CI-\]\[[Co{{(N{{H}_{3}})}_{4}}BrCI]Br\] (Ionisation isomers)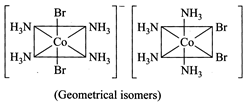 (3) Pd has \[4{{d}^{8}}\]configuration which has higher CFSE and thus the complex is square planar and diamagnetic. In some ligands, like ambidenate ligands, there are two possible coordination sites. In such cases, linkage isomerism exist, e.g., \[N{{O}_{2}}\]group can be bonded to metal ions through nitrogen \[(-N{{O}_{2}})\]or through oxygen \[(-ONO).\]
(3) Pd has \[4{{d}^{8}}\]configuration which has higher CFSE and thus the complex is square planar and diamagnetic. In some ligands, like ambidenate ligands, there are two possible coordination sites. In such cases, linkage isomerism exist, e.g., \[N{{O}_{2}}\]group can be bonded to metal ions through nitrogen \[(-N{{O}_{2}})\]or through oxygen \[(-ONO).\]
You need to login to perform this action.
You will be redirected in
3 sec
