A) \[MnO_{4}^{-}\]
B) \[M{{n}^{2+}}\]
C) \[C{{l}_{2}}\]
D) \[C{{r}^{3+}}\]
Correct Answer: A
Solution :
[a] Most positive \[E{{{}^\circ }_{red}}/\] value means most oxidising agent Most negative \[E{{{}^\circ }_{red}}\] value means most reducing agent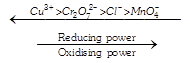
You need to login to perform this action.
You will be redirected in
3 sec
