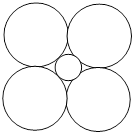
A) 223 pm
B) 224 pm
C) 181 pm
D) 362 pm
Correct Answer: D
Solution :
[d] \[\sqrt{2a}=4{{r}_{C{{I}^{-}}}}\] \[4{{r}_{C{{I}^{-}}}}=\frac{a\sqrt{2}}{4}=\frac{a}{2\sqrt{2}}=\frac{512}{2\sqrt{2}}=361.8pm\cong 362pm\]You need to login to perform this action.
You will be redirected in
3 sec
