A) \[\frac{{{a}^{3}}}{27}\]
B) \[\frac{{{a}^{3}}}{64}\]
C) \[\frac{{{a}^{3}}}{2\sqrt{2}}\]
D) \[\frac{{{a}^{3}}}{8}\]
Correct Answer: D
Solution :
[d]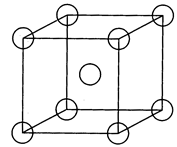 The centre atom of I will be at corner of 2. Hence cube 2 divide volume by 8. \[\therefore \frac{{{a}^{3}}}{8}=\]Common volume?
The centre atom of I will be at corner of 2. Hence cube 2 divide volume by 8. \[\therefore \frac{{{a}^{3}}}{8}=\]Common volume?
You need to login to perform this action.
You will be redirected in
3 sec
