A) \[{{X}_{5}}{{Y}_{4}}{{Z}_{8}}\]
B) \[{{X}_{8}}{{Y}_{4}}{{Z}_{5}}\]
C) \[{{X}_{2}}Y{{Z}_{2}}\]
D) \[XY{{Z}_{2}}\]
Correct Answer: A
Solution :
[a] Number of X-atoms in unit cell = 4 (ccp) Number of Y-atoms=4 Number of Z-atoms=8 If atoms along one body diagonal are removed then 2X, 1Y and 2Z atoms will be absent. Effective number of atoms may be calculated as \[X=4-\left( 2\times \frac{1}{8} \right)=\frac{15}{4}\] \[Y=4-1=3\] \[z=8-2=6\] Simplest ratio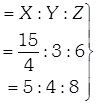 Simplest formula \[={{X}_{5}}{{Y}_{4}}{{Z}_{8}}\]
Simplest formula \[={{X}_{5}}{{Y}_{4}}{{Z}_{8}}\]
You need to login to perform this action.
You will be redirected in
3 sec
