A) \[\left[ FeC{{l}_{3}} \right]F{{e}^{3+}}\] \[O{{H}^{-}}\]
B) \[\left[ Fe{{(OH)}_{3}} \right]F{{e}^{3+}}\] \[C{{I}^{-}}\]
C) \[\left[ Fe{{(OH)}_{3}} \right]F{{e}^{3+}}\] \[3C{{I}^{-}}\]
D) \[\left[ FeC{{l}_{3}} \right]F{{e}^{3+}}\] \[3O{{H}^{-}}\]
Correct Answer: C
Solution :
[c] \[Fe{{(OH)}_{3}}\] Sol adsorbs common ion of \[FeC{{l}_{3}}\] and \[Fe{{(OH)}_{3}}\] from \[FeC{{l}_{3}}\], i.e. \[F{{e}^{3+}}\]and positively charged sol is the fixe part of the electrical double layer. Thus, \[C{{I}^{-}}\](to balance charge of \[F{{e}^{3+}}\]) ions are on the mobile part of the electrical double layer.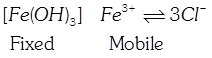
You need to login to perform this action.
You will be redirected in
3 sec
