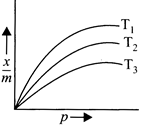
A) \[{{T}_{1}}<{{T}_{2}}<{{T}_{3}}\]
B) \[{{T}_{1}}={{T}_{2}}={{T}_{3}}\]
C) \[{{T}_{3}}<{{T}_{2}}<{{T}_{1}}\]
D) No specific order
Correct Answer: A
Solution :
[a] Adsorption is an exopthermic change thus, as temperature increases, extent of adsorption decreases. Extent of \[\left( \frac{x}{m} \right)\] is minimum at \[{{T}_{3}}\] and is maximum at \[{{T}_{1}}\] Thus, \[{{T}_{1}}<{{T}_{2}}<{{T}_{3}}\]You need to login to perform this action.
You will be redirected in
3 sec
