A) All \[p\pi \]-\[p\pi \] bonds
B) one \[p\pi \]-\[p\pi \] and two \[p\pi \]-\[p\pi \] bonds.
C) one \[p\pi \]-\[p\pi \] and one \[p\pi \]-\[p\pi \] bonds
D) two \[p\pi \]-\[p\pi \] and one \[p\pi \]-\[p\pi \] bonds
Correct Answer: D
Solution :
[d] In gas phase, \[S{{O}_{3}}\] molecule is triangular planar having \[s{{p}^{2}}\]-hybridisation of the S-atom. There are three sigma bonds formed by \[s{{p}^{2}}\]-P overlap and three pi-bonds are by one \[p\pi -p\pi \]overlap and two \[p\pi -d\pi \]overlap.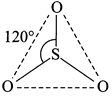
You need to login to perform this action.
You will be redirected in
3 sec
