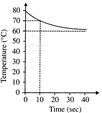
A) 40 and 2
B) 40 and 4
C) 20 and 4
D) 20 and 2
Correct Answer: B
Solution :
[b] Evidently the initial temperature of the water contained in the vessel (Mg) is\[80{}^\circ C\], and the temperature of the water passed into it is\[60{}^\circ C\], as the final temperature of the mixture tends to attain a value of \[60{}^\circ C\]. \[M\times 1(80-70)=m\times 10\times 1(70-60)\] or, \[M/m=10\] Since the heat exchanged after a long time is 800 cal. \[(Mg)(1cal/m{}^\circ C)(80-60{}^\circ C)=80cal\] Or, \[M=40g\text{ }\therefore \,\,\,\,m=4g\]You need to login to perform this action.
You will be redirected in
3 sec
