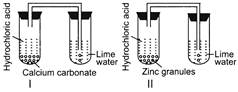
A) The gas coming out from test tube I turned lime water milky.
B) The gas coming out from test tube II turned lime water milky.
C) The gases coming out from both the test tubes turned lime water milky.
D) None of these.
Correct Answer: A
Solution :
\[\underset{\begin{smallmatrix} Calcium \\ carbonate \end{smallmatrix}}{\mathop{CaC{{O}_{3}}}}\,+\underset{\begin{smallmatrix} Hydrochloric \\ acid \end{smallmatrix}}{\mathop{2HCl}}\,\to \underset{\begin{smallmatrix} Calcium \\ chloride \end{smallmatrix}}{\mathop{CaC{{l}_{2}}}}\,+\underset{\begin{smallmatrix} Carbon \\ dioxide \end{smallmatrix}}{\mathop{C{{O}_{2}}}}\,+\underset{Water}{\mathop{{{H}_{2}}O}}\,\] Carbon dioxide gas turns lime water milky. \[\underset{\begin{smallmatrix} Carbon \\ dioxide \end{smallmatrix}}{\mathop{C{{O}_{2}}}}\,+\underset{\begin{smallmatrix} Lime \\ water \end{smallmatrix}}{\mathop{Ca{{(OH)}_{2}}}}\,\to \underset{\begin{smallmatrix} Calcium \\ carbonate \\ (milky) \end{smallmatrix}}{\mathop{CaC{{O}_{3}}}}\,+{{H}_{2}}O\] \[\underset{Zinc}{\mathop{Zn}}\,+\underset{\begin{smallmatrix} Hydrochloric \\ acid \end{smallmatrix}}{\mathop{2HCl}}\,\to \underset{\begin{smallmatrix} Zinc \\ chloride \end{smallmatrix}}{\mathop{ZnC{{l}_{2}}}}\,+\underset{\begin{smallmatrix} Hydrogen \\ gas \end{smallmatrix}}{\mathop{{{H}_{2}}}}\,\] \[{{H}_{2}}\] gas does not lime water milky.You need to login to perform this action.
You will be redirected in
3 sec
